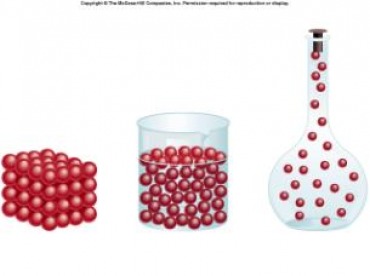
Kinetic Molecular Theory !
- In 1860 Ludwig Boltzman and James Maxwell each proposed a model to explain the properties of gases. This model makes several assumptions about the size, motion and energy of gas particles.
- Objects in motion have energy called Kinetic Energy.
- The Kinetic Molecular Theory describes the behavior of gases in terms of particles in motion.
1. Particle Size :
- Gases consist of small particles that are separated from one another by empty space. Because gas particles are far apart, there are no significant attractive or repulsive forces among them.
- Gas particles are in constant random motion. Collisions between gas particles are elastic. Elastic Collision is one in which no kinetic energy is lost. Kinetic Energy may be transferred between colliding particles but the total kinetic energy of 2 particles does not change.
- Two factors determine the kinetic energy of a particle, these two factors are mass and velocity.